Online Course Support
go to WEEK 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 MSE 5090: Case Studies in Material Selection

Student Case Study Guidelines - Example case study from Handbook of Case Histories in Failure Analysis1
Contents of this page:
Case Study: Degradation of a Main Combustion Chamber Liner on a Space Shuttle Main Engine Degradation of a Main Combustion Chamber Liner on a Space Shuttle Main Engine
J.H. Sanders and P.S. Chen, IIT Research Institute, Marshall Space Right Center, Alabama R.A. Parr, National Aeronautics and Space Administration, Marshall Space Right Center, Alabama
Pinhole defects were found in a main combustion chamber mode from NARIay-Z after an unexpectedly short time in service. Analysis indicated that the throat section of the liner had been exposed to very severe environmental conditions of high temperature and high oxygen content, which caused ductility loss and grain-boundary separation. The excessive oxygen content in the liner was attributed to diffusion from an oxygen-rich environment that had resulted from nonuniform mixing of propellants. The internal oxygen embrittled the alloy and reduced its thermal conductivity, which resulted in a higher hot-gas wall temperature and associated degradation of mechanical properties.
Key Words Copper-base alloys Combustion chambers Liners Transgranular fracture Intergranular fracture Internal oxidation Pinholes Engine components Rocket engines Reentry vehicles Alloys Copper-base alloy—NARloy-Z
Background Pinhole defects were found in a main combustion chamber made from NARloy-Z after an unexpectedly short time in service. Applications The main combustion chamber (MCC) liner is a critical part of the space shuttle main engine (SSME). It is designed to contain the high-temperature reaction between liquid hydrogen (LH2) and liquid oxygen (LOX) that produces the thrust required for propulsion. To maintain low operating temperatures, NARloy-Z, a material with high thermal conductivity, is used for the liner, with cooling channels machined into it through which LH2 is pumped (Fig. 1). NARloy-Z is a copper-base alloy containing 3% Ag and 0.5% Zr. Silver acts as a precipitation hardener, and zirconium acts as a getter for soluble oxygen. The NARloy-Z goes through a fabrication process that includes casting, forging, and heat treatment. Chemical analysis is performed at various stages in the fabrication process to ensure that impurity levels are minimal. Cooling channels are machined into the liner and filled with wax. An electroforming process is then used to apply structural nickel to the exterior of the liner. The SSME is designed to operate in a reducing fuel-rich environment, as a hyperstoichiometric mixture of hydrogen to oxygen is injected into the MCC.
Fig. 1 Schematic of the main combustion chamber in the space shuttle main engine. (a) Profile of the MCC and the location of pinholes
.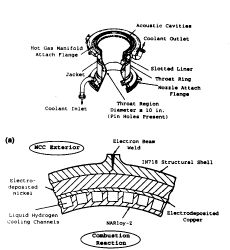
(b) Cross section showing the location of the cooling channelsCircumstances
leading to
failureAn unacceptable number of pinholes developednear the throat of the MCC liner after a short time, allowing excessive hydrogen to leak into the combustion chamber.
These pinholes were confined to an arc of less than 50 mm (2 in.) along the 810 mm (32 in.) circumference of the throat.
Pertinent
specificationsThe normal hot-gas wall temperature and stag nation pressure near the throat are estimated to be 540 °C (1000 °F) and 20.7 MPa (3009 psia), respectively.
The coolant (LH2) inlet and exit pressures are 41 and 31.3 MPa (5949 and 4543 psia), respectively. The maximum oxygen concentration in NARloy-Z is 75 ppm.
Performance
of other parts
in same or
similar serviceThe MCC under investigation failed after eight firings for a total of 3000 s. This was significantly less than the average lifetime of approximately
30,000 s. An MCC is retired from service when the hydrogen coolant loss is greater than the engine system balance can accommodate.
Specimen
selectionSpecimen locations were selected to determine the nature and extent of material degradation. Specimens were sectioned from the perimeter of the throat revealing the cross-sectional area
(hot-gas wall to electrodeposited copper) with maximum material degradation. For comparison, sections were also taken away from the throat where no apparent degradation was observed.
Visual
Examination
of General
Physical
FeaturesSpecimens taken from the throat section at the served as some of the channels appeared rounded at location of maximum degradation exhibited dark the edge near the hot-gas wall. Other specimens,
bands that ran radially between the hot-gas wall both in the throat region and away from the throat, and the cooling channels. Deformation was ob- had no visible abnormalities.
Fig. 2 Optical micrographs of grain-boundary
is atseparation in the throat section. The hot-gas wall
the top.Testing
Procedure
and Results
(a) Massive deformation forms pin-holes. 42.5x.

(b) Minimal grain-boundary separation. 85x
Surface
examinationOptical microscopy revealed that the dark bands noted in the visual examination contained dark secondary phases and dark precipitates in the matrix. The pinholes appeared to be the result of grain-boundary separation, which propagated from the cooling channel toward the hot-gas wall in cooling channels that had severely rounded edges (Fig. 2). Microprobe analysis was used to determine the morphology and composition of the matrix and phases. Typical microstructure of NARloy-Z was observed away from the banded region (Fig. 3). The second-phase intermetallic located at the grain boundaries had a composition of Cu-10Ag-22Zr. The small silver precipitates that provide precipitation hardening were not visible.
Figure 4 shows an area within the banded region. Extensive grain-boundary separation had occurred (Fig. 4a), and the intermetallic had dissociated and appeared heterogeneous (Fig. 4b). The composition of the dissociated intermetallic ranged from 52 to 60% Cu, 12 to 15% Ag, and 27 to 32% Zr. Precipitates were also observed in the banded region, having a more homogeneous appearance (Fig. 5) with a composition of 38 to 41% Cu, 14 to 16% Ag, and 35 to 38% Zr. The silver precipitates were also observed in the banded region, with a rodlike structare and preferred orientation.
Fig. 3 Backscatter electron micrograph of the MCC away from the hot-gas wall, showing normal microstructure with clean grain boundaries and second-phase intermetallics with a composition of Cu-10Ag-22Zr. 236x
Fig. 4 Micrographs of the banded region of the MCC.

(a) Cracking and grain-boundary spearation, backscatter mode. 296x.
(b) Dissociated intermetallics with compositions of 52 to 60% Cu, 12 to 15% Ag, and 27 to 32% Zr. 1480xFig. 5 Micrographs of the banded region of the
that have compositions of 52 to 60% Cu,MCC where homogeneous phases predominate 27 to 32% 12 to 15% Ag, and 27 to 32% Zr. 
(a) Backscatter mode. 1000x.
(b) Secondary mode. 2000xTransmission electron microscopy (TEM)
was used to determine the microstructure of the silver precipitates. Figure 6(a) shows the size and distribution in the bulk material, away from the hot-gas wall and banded region. Figure 6(b) shows
a similar structure near the hot-gas wall. As shown, the silver precipitates coarsened near the hot-gaswall.Selected-area diffraction patterns revealed that the precipitates in Fig. 6(a) were sernicoherent, whereas those near the hot-gas wall (Fig. 6b) were incoherent.The microstructure in the banded region is shown
in Fig. 7. Precipitates were semicoherent and arranged along deformation bands.Fig. 6 TEM micrographs of silver precipitates in the copper interface where matrix taken from a location near the electrodeposited
precipitates are semicoherent
(a), and the hot-gas wall where precipitates are incoherent
(b). Coherency determined by selected-area diffraction patterns. 53,000xFig. 7 TEM micrographs of the banded region, semicoherent silver showing deformation bands together with
precipitate rearrangement.
(a) 22,000x.

b) 70,000x
Chemical
analysis/
IdentificationMicroprobe analysis showed an increase in soluble oxygen in the banded region of the matrix. The intensity of the oxygen peak increased with the amount of zirconium in the intermetallics. In sum-mary oxygen content in a typical intermetallic (Fig. 3) was very low, whereas it was higher in the
dissociated intermetallic in the banded region (Fig. 4) and was the highest in the high-zirconium phase shown in Fig. 5.
Bulk chemical analysis was performed on the material using combustion analysis. Throat sec-tions of the MCC had an average oxygen level of 131 ppm.
Simulation tests A limited database exists on NARIoy-Z, because the material was designed specifically for the
SSME and is not used commercially. Th provide semiquan-titative estimation of the temperature that the liner experienced, experiments were performed on non-degraded sections of the MCC, exposing specimens to temperatures from 540 to 980 0C (1000 to 1800 °F) in air and vacuum (Ref 1). These experiments re-vealed that the dissociation
of intermetallics oc-curred in the presence of oxygen diffusing into the material. Zirconium
reacted with internal oxygen to form ZrO2 and free up silver. A transformed zone was produced on the surface of the specimens, with a thickness in agreement with the theoretical penetration distance of oxygen in copper. Silver precipitates remained semicoher-ent at 540 °C (1000 °F) (average diameter <20 nm), whereas complete incoherency and coarsening were observed above 760 0C (1400 °F) (average diameter
>40 nm).
Discussion The high-oxygen phases in the banded region were of two different types. The phases shown in Fig. 4 appeared similar to the dissociated intermet-allics observed in the transformed surface region ob-tained during simulated tests (Ref 1). The other phase found in the banded region appeared more ho-mogeneous (Fig. 5). The overall zirconium and oxy-gen contents in the homogeneous phase were higher than in the dissociated intermetallics. Based on a study on the dispersion strengthening of copper-zir-conium alloys by internal oxidation (Ref 2), the ho-mogeneous phase was produced by the rapid move-ment of the oxidation front through the material. This phenomenon reduced the time necessary for substantial zirconium diffusion away from the origi-nal intermetallic location. The rodlike silver precipitates within the matrix of the banded region (Fig. 7) occur from high- temperature deformation, which was the result of the pressure gradient in the weakened liner material. The high temperature facilitated the rearrange-ment of silver precipitates along low-energy sites on deformation bands. Similar structure has been re-ported in other investigations of copper-base alloys (Ref 3, 4).
The effect of strain and high levels of oxygen in the material created a condition susceptible to grain-boundary separation at elevated tempera-tures. Grain-boundary decohesion occurred as ox-ides formed at grain boundaries, reducing inter-facial energy (Ref 5, 6). The failure mode changed from ductile transgranular to brittle intergranular. Void formation was enhanced in the presence of grain-boundary precipitates such as oxides (Ref 7-9). Continued coalescence of these voids increased the likelihood of grain-boundary failure. The TEM micrographs of the silver precipitates in the MCC liner were used to estimate the temperatures encountered by the MCC during service. Comparison of precipitate size to those produced in simulated tests revealed that the hot-gas wall temperature was in excess of 760 °C (1400 °F) and perhaps as high as 870 °C (1600 °F). The temperature increase at the hot-gas wall resulted from a decrease in cooling efficiency as the NARloy-Z micro-structurewas transformed by high levels of oxygen. The incoherent nature of silver precipitates reduced the strength of the material, causing the liner to fail prematurely.
Conclusion and RecommendationsMost probable cause It was concluded that the MOO was exposed to more severe environmental conditions than normal, most probably the result of nonuniform mixing of fuel and oxidizer. Because the degradation was observed in only a particular region, the oxygen-to-hydrogen ratio must have been
nonuniform along the circumference of the
MCC.
The environmental conditions produced an excessive amount of internal oxygen and temperatures higher than 760 °C (1400 °F), which altered the microstructure of the material, resulting in reduced grain-boundary and matrix strength.
Remedial
actionAbnormalities in the mixing of fuel and oxidizer that allow oxygen to come into contact with the hot-gas wall at the throat should be eliminated to in-crease service life. Transient conditions that accompany
engine startup and shutdown must be carefully scrutinized to ensure that a hyperstoichiometric mixture of fuel to oxygen is maintained.
References 1. J.H. Sanders and P.S. Chen, “Microstructure Evalu-ation of Undoped and Oxygen Doped NARIoy-Z at High Temperatures,” Report No. IITRI-P06150-P495A, IITRI/MRF, NASA — Marshall Space Right Center, 27 Dec 1991 2. J.H. Swisher and E.O. Fuchs, Dispersion-Strengthening of Copper by Internal Oxidation of Two-Phase Copper-Zirconium Alloys, Copper and Its Alloys, Monograph and Report Series No. 34, The Institute of Metals, London, 1970, p 292-296
3. N.E. Paton and WM. Robertson, “Progress Report on the Metallurgy of NARloy-Z,” North American Rock-well Center, 15 March 1973
4. K.S. Yu and WD. Nix, “Final Report on a Study of the High Temperature Creep Behavior of NARloy-Z,” Rockwell International Corp., Sept 1985
5. T.G. Nieh and WD. Nix, Embrittlement of Copper Due to Segregation of Oxygen to Grain Boundaries, Metall. Trans A, Vol 12A, 1961, p 893-901 6. W.R. Opie, P.W. Taubenblat, and Y.T. Hsu, A Funda-mental Comparison of the Mechanical Behavior of Oxygen-Free and Tough-Pitch Coppers, Copper and Its Alloys, Monograph and Report Series No. 34, The Institute of Metals, London, 1970, p106-110
7. H.E. Evans, Cavity Formation and Metallurgical Changes Induced by Growth of Oxide Scale, Mater. Sci. Technol., Vol 4, Dec 1988, p 1089-1098
8. 5. Sato, T. Otsu, and E. Hata, Embrittlement of Cop. per Alloys During Annealing, Copper and Its Alloys, Monograph and Report Series No. 34, The Institute of Metals, London, 1970, p 135-141
9. R.T. Chen and J.R. Weertman, Grain Boundary Cavi-tation in Internally Oxidized Copper, Mater Sci. Eng., Vol 64,1984, p 15-25
1. Esaklul, K., Edt., Handbook of Case Histories in Failure Analysis. ASM International®, Materials Park, OH. 1992, pp.56-60.


Last update 10-4-98